
TURNBULL LAB


Polydnavirus life cycle: Polydnaviruses (PDVs) are integrated into a host wasp genome; all members of the wasp species are infected with the same PDV species. Expression of PDV genes in the infected (parasitized) host caterpillar disrupts host immunity and development. PDV genes are thus a (the?) primary driver of host susceptibility to parasitization.

Proposed model of Vinnexin disruption of hemocytic encapsulation. Vinnexins may alter gap junctional activity, disrupting morphogen gradient through one of several means including loss of voltage differences, and (C) by interacting with adhesion and signaling molecule distribution and altering their distribution.

Live cell imaging of CsIV VnxD as a fusion with mCherry.
Polydnavirus Innexins
Polydnaviruses are highly unusual viruses: they are obligate mutualists of some braconid and ichneumonid parasitoid wasps. During parasitization of a caterpillar host, polydnavirus is delivered along with the wasp egg. Virus infection and subsequent gene expression results in multiple immune, metabolic and developmental pathologies. Absent virus gene expression, the caterpillar typically recognizes the wasp offspring with fatal consequence for the latter. As such, better understanding of polydnavirus genes can provide insight into parasitoid wasp host range dynamics.
In the Turnbull lab, we are studying the functional and evolutionary physiology of the vinnexin gene family from the polydnavirus CsIV. Vinnexins are homologues of Innexins, which are the structural units of gap junctions in invertebrates including insects. The Vinnexins form functional gap junctions, and have differential electrophysiological, cellular and organismal characteristics. We are currently investigating the consequence of vinnexin expression in insects using various recombinant approaches, coupled to bioassays, and biochemical and cellular techniques.

(Above) Co-expression and detection of Inx2 from the caterpillar Spodoptera frugiperda, Sf-Inx2, with CsIV VnxD demonstrates that the molecules exhibit extensive co-localization in Sf9 cells. From Hasegawa and Turnbull 2014.

(Right) Immunomicroscopy of CsIV VnxG following expression in Drosophila melanogaster (using Act5C-Gal4 > UAS-vnxg). VnxG-HA localized to embryo cell membranes as expected and resulted in significant embryonic mortality. From Hasegawa et al 2017.
Bioelectricity in Insect Biology
Bioelectric characteristics of cells, membrane potential in particular, are outputs of diverse physiological processes, while they also have been shown to drive physiological processes. The Turnbull lab is investigating the basis for and patterns of bioelectricity in three principle insect processes: ontogeny and regeneration, immunity, and interaction with pathogens. We have found that in all three processes, membrane potential patterns reflect changes in cell physiology, and are currently testing necessity of this relationship.


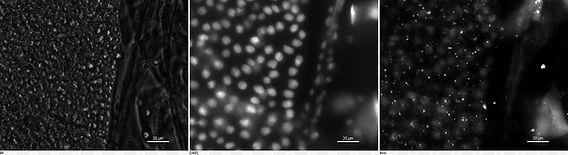
(Top left) bright-field image of 4th instar Heliothis virescens caterpillar mid gut cells. (Top right) DiBac(4)3 stained 4th instar H. virescens caterpillar mid guts cell under fluorescence light.